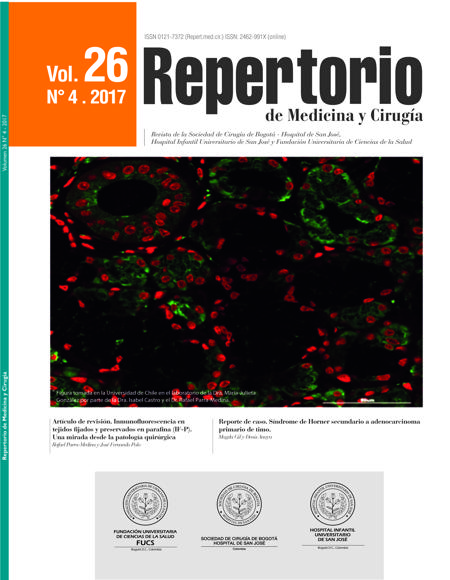
Immunofluorescence labelling of paraffin-fixed and embedded tissue sections (IF-P). From the surgical pathology perspective
Inmunofluorescencia en tejidos fijados y preservados en parafina (IF-P). Una mirada desde la patología quirúrgica
![]()
![]()

Show authors biography
The objective of immunofluorescence labelling of paraffin-fixed and embedded tissue sections (IF-P), as well as of immunohistochemistry (IHC) and immunofluorescence (IF) staining, is to detect the presence of antigens using the antigen-antibody interaction. This technique is little- known in surgical pathology for it has primarily been used in experimental studies. This article aims to review the basic concepts of IF-P, and identify its advantages and disadvantages compared with IF and IHC, and its possible applications in the surgical pathology field.
Article visits 1117 | PDF visits 632
Downloads
1. Perkel JM. Immunohistochemistry for the 21st Century. Science. 2016;351:1098–100.
2. Mason DY, Micklem K, Jones M. Double immunofluorescence labelling of routinely processed paraffin sections. J Pathol. 2000;191:452–61.
3. Spector DL, Goldman RD. Basic methods in microscopy protocols and concepts from cells: A laboratory manual. Cold Spring Harbor, New York, NY, USA: Cold Spring Harbor Laboratory Press; 2006.
4. Dabbs DJ. Diagnostic immunohistochemistry. 3 rd ed. Philadelphia, PA: Elsevier/Saunders; 2010.
5. Niki H, Hosokawa S, Nagaike K, Tagawa T. A new immunofluorostaining method using red fluorescence of PerCP on formalin-fixed paraffin-embedded tissues. J Immunol Methods. 2004;293:143–51.
6. Bataille F, Troppmann S, Klebl F, Rogler G, Stoelcker B, Hofstadter F, et al. Multiparameter immunofluorescence on paraffin-embedded tissue sections. Appl Immunohistochem Mol Morphol. 2006;14:225–8.
7. Robertson D, Savage K, Reis-Filho JS, Isacke CM. Multiple immunofluorescence labelling of formalin-fixed paraffin-embedded (FFPE) tissue. BMC Cell Biol. 2008;9:13.
8. Pan J, Thoeni C, Muise A, Yeger H, Cutz E. Multilabel immunofluorescence and antigen reprobing on formalin-fixed paraffin-embedded sections: Novel applications for precision pathology diagnosis. Mod Pathol. 2016;29:557–69.
9. Messias NC, Walker PD, Larsen CP. Paraffin immunofluorescence in the renal pathology laboratory: More than a salvage technique. Mod Pathol. 2015;28:854–60.
10. Singh G, Singh L, Ghosh R, Nath D, Dinda AK. Immunofluorescence on paraffin embedded renal biopsies: Experience of a tertiary care center with review of literature. World J Nephrol. 2016;5:461–70.
11. Gown AM. Diagnostic immunohistochemistry: What can go wrong and how to prevent it. Arch Pathol Lab Med. 2016;140:893–8.
12. Lin F, Chen Z. Standardization of diagnostic immunohistochemistry: Literature review and Geisinger experience. Arch Pathol Lab Med. 2014;138:1564–77.
13. Parra-Medina R, Morales SD. Diagnostic utility of epithelial and melanocitic markers with double sequential immunohistochemical staining in differentiating melanoma in situ from invasive melanoma. Ann Diagn Pathol. 2017;26:70–4.
14. Erben T, Ossig R, Naim HY, Schnekenburger J. What to do with high autofluorescence background in pancreatic tissues — an efficient Sudan black B quenching method for specific immunofluorescence labelling. Histopathology. 2016;69:406–22.
15. Viegas MS, Martins TC, Seco F, do Carmo A. An improved and cost-effective methodology for the reduction of autofluorescence in direct immunofluorescence studies on formalin-fixed paraffin-embedded tissues. Eur J Histochem. 2007;51:59–66.
16. Baschong W, Suetterlin R, Laeng RH. Control of autofluorescence of archival formaldehyde-fixed, paraffin-embedded tissue in confocal laser scanning microscopy (CLSM). J Histochem Cytochem. 2001;49:1565–72.
17. Ursini-Siegel J, Beauchemin N, editores. The Tumor environment. Methods and protocols. Humana Press/Springer; 2016.
18. Wolff AC, Hammond MEH, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013.
19. Rüschoff J, Dietel M, Baretton G, Arbogast S, Walch A, Monges G, et al. HER2 diagnostics in gastric cancer-guideline validation and development of standardized immunohistochemical testing. Virchows Arch. 2010;457:299–307.
20. Giefing M, Siebert R. FISH and FICTION to detect chromosomal aberrations in lymphomas. Methods Mol Biol. 2013;971:227–44.
21. Parra-Medina R, Mayayo E. ¿Hacia dónde vamos con la patología moderna? La patología personalizada. Rev Esp Patol. 2016;49:205–7.
22. Sheffield BS. Immunohistochemistry as a practical tool in molecular pathology. Arch Pathol Lab Med. 2016;140: 766–9.